How the least bit of Global Warming Causes so much Climate Change
The Earth’s atmosphere has been steadily heating up for the past two centuries, about 1.3 degrees Fahrenheit since 1906, according to the IPCC.[i] That’s 0.7˚ Celsius for our non-American readers. Which does not really seem like much, does it? If it’s 65˚F on a comfortable October afternoon, would it really feel much different if it were 66.3˚F. I don’t think so. And yet that little bit of warming is being blamed for floods and droughts, heat waves, crop losses, climate refugees, ecosystem disruptions, increase in the frequency and intensity of storms, and of course the melting of glaciers and ice caps and rising sea levels. So what gives? How does a little increase in temperature lead to such dire climatic consequences?
We’ll use logic and the simplest of math to make sense of it. First the logic. When we speak of a 1.3 degree rise in temperature, we mean that every place on the Earth is that much warmer all the time; every second of every day of every year. And not just on the surface of the Earth, but for the entire bottom seven miles of the atmosphere, as well. That, we’ll see, represents a lot of energy.
Secondly, temperature and energy are not the same phenomenon. They tell you different things. For instance, temperature can give you a sense of how high your fever has risen and therefore how sick you may be. However, when you want to know how much energy your food will provide or how much work you did during your run, you measure these in calories, or joules if you are a scientist. The 1.3 degree Fahrenheit rise in temperature since 1906 only hints at the true extent of the energy that the Earth has been absorbing. It is the energy in a physical system, such as the atmosphere, that tells you how much work can be done and therefore the magnitude of the possible consequences. Temperature cannot move wind and waves, but energy can.
So, now for some simple math. To give us a sense of how much energy a 1.3 degree rise represents, we rely on a simple formula: q = mc∆T. Now please don’t run away. It is true that math education in the United States is so torturous, so removed from any practical uses, and therefore so meaningless, that educators have made generations of people feel frustrated and stupid for not understanding it, and then most of these people hate math for making them feel this way. Still, we can appreciate the wonders that even simple mathematics can reveal. So stay with me for a moment.
q is the heat energy measured in calories or joules that a system gains or loses. The system can be a rock, a lake, or the atmosphere, whatever you are studying.
m is the mass of that system. A one-degree rise in a lake is going to translate into a lot more energy than a one-degree rise in a bucket of water.
c is something call specific heat. Different substances absorb heat differently. It takes way more energy to raise a kilogram of water one degree Fahrenheit than it does a kilogram of iron, believe it or not. Just think how hot a car’s metal skin gets in the summer and how cold in the winter. Metal’s low specific heat tells you that a little heat quickly raises its temperature.
∆T just means the change in temperature.
So, we’re going to use this equation q = mc∆T to give us a sense of the energy the atmosphere has acquired since 1906.
Since we know the temperature change we’re considering (0.7˚C, because we’re using the metric system) and the specific heat of air (1004 J/kg·K), we need to find only the mass of the Earth’s atmosphere. The atmosphere’s mass is estimated to be about 5.10 x10^18 kg. We will use only 80% of that mass, because it is the bottom layer of the atmosphere that is warming. This layer, where our weather occurs, is called the troposphere and contains about 80% of the atmosphere’s molecules.[ii] 80% of 5.10 x10^18 kg equals 4.08 x 10^18 kg. By the way, 10^18 is a million trillion or 1,000,000,000,000,000,000 -- a very big number. The Earth is big and we are very small.
So, the additional energy in the atmosphere associated with a 0.7˚C change =
q = mc∆T = (4.08 x 10^18 kg)(1004 J/kg·K) (0.7˚C) = 2.9 x 10^21 J.
10^21 is clearly an enormous number – a billion trillion. For comparison, the atomic bomb “Little Boy” that was dropped on Hiroshima in 1945 released 63 trillion joules (6.3 x 10^13 J) of energy.[iii] This means that the energy added to the atmosphere represented by a 0.7˚C temperature rise equals the energy that would be released by 46 million (46 x 10^6) Hiroshima-sized explosions. These comparisons are for illustrative purposes only, as the energy released in hyper-energetic nuclear events will behave differently than when that same energy is held in the molecules of the atmosphere. However, even in this enormous volume of air, the energy is not spread evenly. It moves about the planet and collects into storms, cyclones, and air masses. We are trying to contemplate unimaginable amounts of energy interacting in unimaginably complex ways! What is simple to understand is this: the portion of the atmosphere where weather and climate occur carries far more energy than it did a mere hundred years ago. How much energy? The energy contained in 46 million atomic bombs. Then, it may not surprise us after all when we witness – mainly through the media – historic changes in weather events, climatic shifts, and in the biosphere.
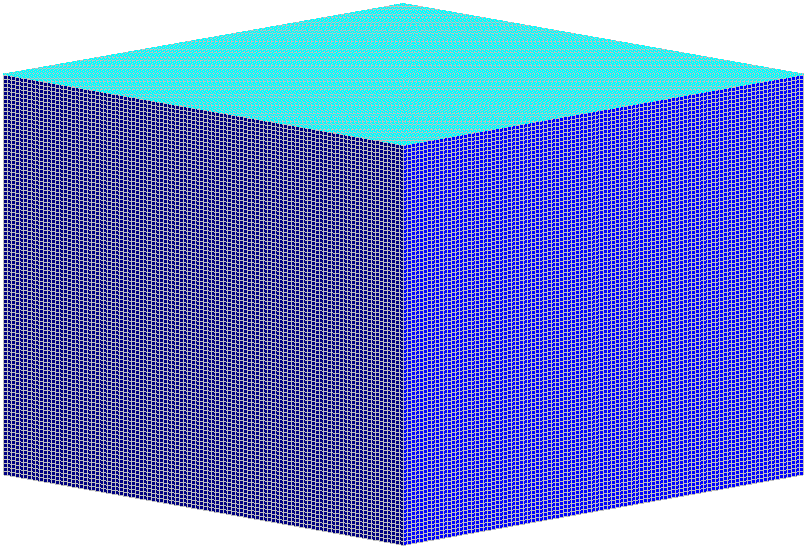
BELOW ARE 46 CUBES, EACH WITH 100 SQUARES TO A SIDE. THEREFORE, THESE 46 CUBES HOLD A TOTAL OF 46 MILLION SQUARES. WHAT?! AND IN OUR LITTLE EXERCISE, EACH SQUARE REPRESENTS ONE ATOMIC BOMB OF ENERGY THAT HAS BEEN ADDED TO THE EARTH’S ATMOSPHERE IN THE LAST 100 YEARS.
Image borrowed from http://gwydir.demon.co.uk/jo/numbers/arab/intro.htm.
And we may not be surprised that most of the changes will not redound in our favor. Agriculture is only a few thousand years old. We have developed our specific breeds of plants and animals to be successful within the very specific environmental conditions of the past few thousand years. The Earth’s climate has been relatively benign in these millennia, and our domesticated varieties are not as genetically varied and therefore as resilient as wild species. Meanwhile, our population has ballooned to over 7.7 billion people, with another four additional billion expected to join us this century.[iv] Civilization and all its security and comforts are based on food security and therefore on a reliable global climate. This is the system with which we are tampering when we add hundreds of billions of tons of carbon dioxide (CO2) and methane (CH4) to the atmosphere. Scientists expect Civilization to increase the Earth’s atmosphere temperature another two to three degrees Celsius by century’s end; that is, another 130 to 200 “Little Boys” worth of energy.[v] It is the energy involved, not the few degrees of temperature, that is truly frightening.
ENDNOTES
[i] IPCC (Intergovernmental Panel on Climate Change) (2007c) Full Report, Fourth Assessment Report, Working Group 1 Report, “The Physical Science Basis.”
[ii] Wallace, J.M., and Hobbs, P.V. (2006) Atmospheric Science: An Introductory Survey. Elsevier, London, UK.
[iii] Energy released in the two atomic bombs (15 kt in Hiroshima and 21 kt in Nagasaki) = 36 kilotons TNT (Pittock, A.B., Ackerman, T.P., Crutzen, P.J., MacCracken, M.C., Shapiro, C.S., Turco, R.P. (1986) Environmental Consequences of Nuclear War Volume I: Physical and Atmospheric Effects, John Wiley & Sons, New York.) = 1.5 x10^14 joules.
[iv] United Nations Department of Economic and Social Affairs (2017) World Population Prospects: The 2017 Revision.
[v] IPCC (Intergovernmental Panel on Climate Change) (2018) Full Report, Sixth Assessment Report.